FDA Guidelines for Imaging Trials: Ensuring Quality and Safety in Clinical Research
In the ever-evolving landscape of clinical research, imaging technologies play a crucial role in drug development and evaluation. The U.S. Food and Drug Administration (FDA) has established comprehensive guidelines to ensure the quality, safety, and efficacy of imaging trials. This article delves into the key aspects of FDA guidelines for imaging trials, providing valuable insights for sponsors, researchers, and healthcare professionals.
Understanding the Importance of Imaging in Clinical Trials
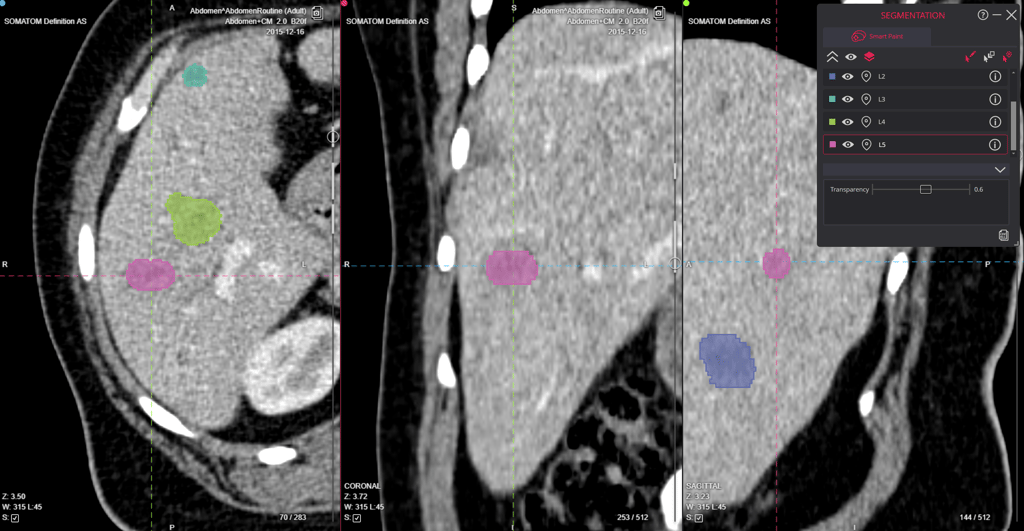
Imaging technologies offer powerful insights into the distribution, binding, and biological effects of pharmaceuticals. According to the FDA's Medical Imaging and Drug Development resource, these technologies contribute important biomarkers and surrogate endpoints, aiding in the understanding of disease progression and the development of new therapies.
If you are looking for a worldwide overview, take a look at Global Regulatory Compliance in Medical Imaging.
FDA's Clinical Trial Imaging Endpoint Process Standards
The FDA has issued a guidance document titled "Clinical Trial Imaging Endpoint Process Standards Guidance for Industry" to assist sponsors in optimizing the quality of imaging data in clinical trials. This guidance focuses on four critical areas:
- Imaging Acquisition: Standards and best practices for capturing high-quality images.
- Display: Guidelines for the proper display of imaging data to ensure accurate interpretation.
- Archiving: Recommendations for the storage and maintenance of imaging data to ensure its integrity and accessibility.
- Interpretation: Standards for the accurate and consistent interpretation of imaging data.
These standards are crucial when imaging is used to assess a trial's primary endpoint or a component of that endpoint. Sponsors can access the full guidance document on the FDA's website.
The Role of Data Monitoring Committees (DMCs) in Imaging Trials
Data Monitoring Committees play a vital role in ensuring the safety of participants and the integrity of data in clinical trials, including those involving imaging endpoints. The FDA has provided comprehensive guidelines for DMCs, which include:
- Primary Responsibilities: Monitoring trial progress, safety data, and critical efficacy endpoints.
- Safety and Efficacy Monitoring: Reviewing accumulating data to identify safety concerns and assess if trial objectives are being met.
- Data Integrity: Ensuring the accuracy and completeness of data collection and management processes.
- Interim Analysis: Conducting analyses at pre-specified points to make informed decisions about trial continuation, modification, or termination.
For detailed information on DMC guidelines, refer to the FDA's Guidance for Clinical Trial Sponsors.
Best Practices for Imaging in Drug Development
The FDA, in collaboration with the National Cancer Institute (NCI), has established several initiatives to promote best practices in imaging for drug development:
- Interagency Oncology Task Force (IOTF): A collaborative effort to share knowledge and resources, facilitating the development of new cancer drugs.
- Joint Fellowship Program: Part of the IOTF initiative, promoting shared expertise and training.
- Critical Path Initiative: Aims to modernize the medical product development process, making it more predictable and less costly.
FDA Guidances for Medical Imaging in Drug Development
The FDA has issued several guidances to assist sponsors in developing medical imaging drugs and biological products:
- Exploratory IND Studies: Provides recommendations for early-phase clinical trials.
- Conducting Safety Assessments: Guidelines for ensuring the safety of imaging agents.
- Clinical Indications: Recommendations for determining the clinical indications for imaging agents.
- Design, Analysis, and Interpretation of Clinical Studies: Best practices for designing and interpreting clinical studies involving imaging technologies.
These guidances can be found on the FDA's Medical Imaging and Drug Development page.
Ensuring Compliance with FDA Guidelines
To ensure compliance with FDA guidelines for imaging trials, sponsors and researchers should:
- Develop a detailed charter outlining DMC responsibilities and procedures.
- Compose DMCs of independent experts with relevant expertise and no conflicts of interest.
- Maintain confidentiality and adhere to blinding procedures to avoid bias.
- Establish clear communication channels between DMCs, trial sponsors, and regulatory authorities.
- Implement predefined criteria for decision-making, prioritizing participant safety and data integrity.
Conclusion
FDA guidelines for imaging trials are designed to ensure the highest standards of quality, safety, and efficacy in clinical research. By adhering to these guidelines, sponsors and researchers can optimize the use of imaging technologies in drug development, ultimately benefiting patients and advancing medical science.
For more information on FDA guidelines and regulations, visit the official FDA website or contact them at 1-888-INFO-FDA (1-888-463-6332).
FAQs
-
What is the primary purpose of FDA guidelines for imaging trials? The primary purpose is to ensure the quality, safety, and efficacy of imaging data in clinical trials supporting drug and biological product approvals.
-
How often should Data Monitoring Committees meet during an imaging trial? The frequency of DMC meetings should be outlined in the trial's charter, with regular meetings scheduled to review data and additional emergency meetings convened if urgent safety issues arise.
-
Can imaging technologies be used as surrogate endpoints in clinical trials? Yes, the FDA recognizes that imaging technologies can contribute important biomarkers and surrogate endpoints, aiding in the understanding of disease progression and therapy development.
-
What is the Critical Path Initiative, and how does it relate to imaging trials? The Critical Path Initiative is an FDA program aimed at modernizing the medical product development process. It includes efforts to improve the use of imaging technologies in drug development and evaluation.
-
Are there specific FDA guidelines for oncology imaging trials? Yes, the FDA collaborates with the National Cancer Institute through the Interagency Oncology Task Force to address specific needs in oncology imaging trials and cancer drug development.
-
How can sponsors ensure data integrity in imaging trials? Sponsors should follow FDA guidelines on data acquisition, display, archiving, and interpretation. Additionally, they should work closely with Data Monitoring Committees to ensure ongoing data integrity throughout the trial.
-
Where can I find the most up-to-date FDA guidelines for imaging trials? The most current guidelines can be found on the FDA's official website, specifically in the sections dedicated to medical imaging and drug development guidance documents.
Pär Kragsterman, CTO and Co-Founder of Collective Minds Radiology
Reviewed by: Rebeca Sanz Barriuso on September 22, 2024