Imaging Core Lab: Advancing Clinical Trials Through Expert Medical Imaging | Complete Guide
An imaging core lab is a specialized facility that provides centralized medical imaging services for clinical trials, ensuring standardized, high-quality image acquisition, processing, and analysis. These labs play a crucial role in drug development, medical device evaluation, and clinical research by providing reliable and consistent imaging data that meets regulatory requirements.
What is an Imaging Core Lab?
The evolution of clinical trials has led to increasingly complex imaging requirements and data management needs. As defined by Applied Clinical Trials:
"The so-called 'core lab' or imaging laboratory for the centralized quality control and assessment of images."
Modern imaging core labs serve as central hubs for managing and analyzing medical images in clinical trials, providing standardized protocols, expert analysis, and quality control measures across multiple research sites and countries.
Essential Technology Infrastructure
At the heart of every successful imaging core lab lies its technology infrastructure. A critical component is the Imaging Clinical Trial Management System (ICTMS), which handles the complex workflow of image acquisition, storage, and analysis. According to the FDA's Clinical Trial Imaging Endpoint Process Standards:
"The purpose of this guidance is to assist sponsors in optimizing the quality of imaging data obtained in clinical trials intended to support approval of drugs and biological products."
Key technology components include:
- Cloud-based image storage and sharing
- Automated workflow management
- Quality control systems
- Regulatory compliance tools
- Multi-site coordination capabilities
Also Read: Imaging Clinical Trial Management Systems (ICTMS): What You Need to Know
Comprehensive Services and Capabilities
The scope of modern imaging core labs extends far beyond simple image storage. As explained by the National Cancer Institute's Cancer Imaging Program:
"Like other types of clinical trials an imaging clinical trial is a research study conducted with people who volunteer to take part. Each study answers specific scientific questions that will determine the value of imaging procedures for detecting, diagnosing, guiding, or monitoring the treatment of disease."
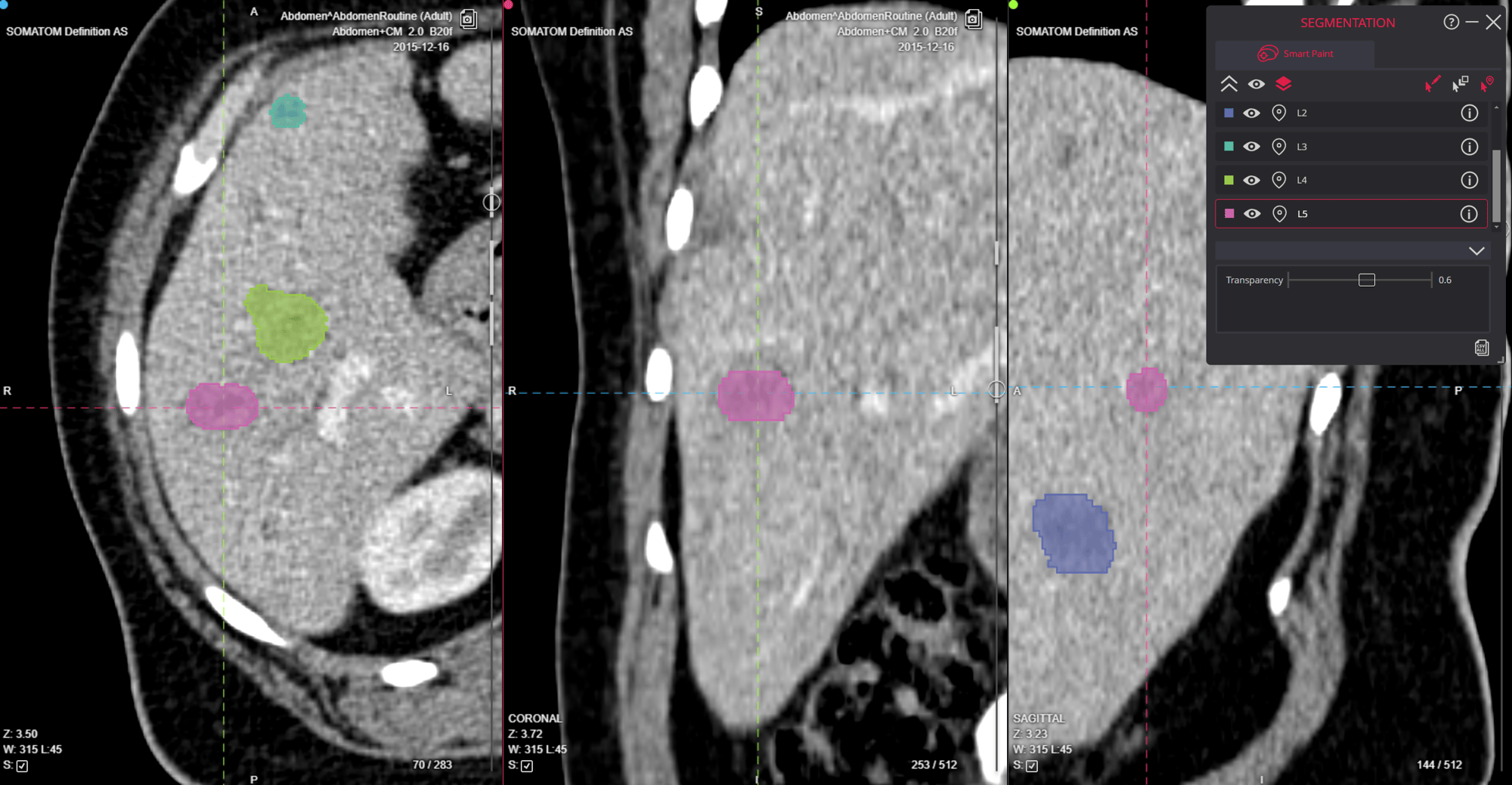
Image Acquisition and Analysis
Clinical trials require different types of imaging expertise depending on their therapeutic focus and endpoints. Modern imaging core labs have evolved to provide specialized services across various therapeutic areas, from oncology and cardiovascular studies to neurology and musculoskeletal research. Each area demands specific expertise in relevant imaging modalities, whether it's MRI for brain studies, CT for tumor assessment, or specialized nuclear medicine techniques for molecular imaging. Core labs maintain teams of therapeutic area experts who understand both the imaging requirements and the clinical context of their specific fields, ensuring that image acquisition and analysis protocols are optimally designed for each trial's unique needs.
Quality Control and Standardization
The FDA's guidance emphasizes the importance of standardized protocols and quality control measures. These guidelines ensure consistency across all trial sites and maintain data integrity throughout the study.
Also Read: Understanding Good Clinical Practice (GCP) in Imaging: An In-Depth Exploration
Market Growth and Industry Impact
The clinical trials imaging sector continues to experience significant growth as the importance of imaging endpoints increases. According to recent market analysis published on BioSpace, the U.S. clinical trial imaging market is projected to reach USD 875.93 million by 2033, growing at a CAGR of 7%.
Also Read: 7 Things Your Imaging CRO Needs in 2024: Staying Ahead in Clinical Trials
Selecting an Imaging Core Lab Partner
When choosing an imaging core lab, organizations must consider their specific therapeutic needs alongside technical capabilities. The ideal partner should demonstrate deep expertise in relevant therapeutic areas, whether it's oncology, cardiovascular, neurology, or other specialized fields. Beyond therapeutic focus, evaluate their technology infrastructure, quality management systems, and global reach capabilities. Look for partners with proven experience in your specific type of trials and therapeutic areas, as this expertise often translates to more efficient study execution and higher quality imaging data.
QMENTA, a provider in the field, emphasizes the importance of:
- Technology Infrastructure
- ICTMS capabilities and features
- Data security measures
- Integration capabilities
- Quality Management
- ISO certifications
- FDA inspection history
- Standard operating procedures
- Global Reach
- Multi-site management capabilities
- International regulatory compliance
- Language support
Video shows the main product features of Collective Minds Research for CROs
Read Also: Collective Minds Research for CROs
Best Practices in Image Management
Successful imaging core labs implement several best practices to ensure optimal outcomes:
- Standardized Protocols
- Consistent image acquisition procedures
- Quality control checkpoints
- Data validation processes
- Staff Training
- Regular protocol updates
- Technical certification requirements
- Ongoing education programs
- Data Management
- Secure storage solutions
- Backup procedures
- Audit trail maintenance
Summary
Imaging core labs continue to evolve as essential partners in modern clinical trials, providing crucial services that ensure high-quality, standardized imaging data for research and regulatory submissions. Their role becomes increasingly important as medical imaging technology advances and clinical trials grow in complexity and global reach.

Collective Minds Research - Tasklist, a simplified view for trial participants, in action.
FAQ
What is the main purpose of an imaging core lab?
An imaging core lab provides centralized medical imaging services for clinical trials, ensuring standardized image acquisition, processing, and analysis across multiple research sites.
Why is an Imaging Clinical Trial Management System (ICTMS) important?
An ICTMS is crucial for managing complex workflows, ensuring data integrity, and maintaining regulatory compliance across multiple trial sites and imaging modalities.
How do imaging core labs ensure data quality?
They implement standardized protocols, provide site training, use specialized software for analysis, and employ expert reviewers to maintain consistent quality across all trial locations.
What role does technology play in modern imaging core labs?
Technology, particularly ICTMS platforms, enables efficient data management, quality control, and collaboration across global trial sites while ensuring regulatory compliance.

Reviewed by: Rebeca Sanz Barriuso on October 31, 2024




