Integrated Core Lab Toolchain: Modern Clinical Trial Imaging Management
Clinical trial imaging management has evolved beyond simple data collection and analysis. Today's integrated core lab toolchain represents a sophisticated ecosystem of interconnected tools, processes, and workflows that streamline clinical trial imaging operations. This comprehensive guide explores how modern toolchains are revolutionizing clinical research efficiency and data quality.
What Is an Integrated Core lab Toolchain?
An integrated core lab toolchain represents the next evolution in clinical trial management, combining various specialized tools and processes into a seamless workflow. This modern approach addresses the growing complexity of multi-center trials and the increasing demand for rapid, accurate data analysis.
An integrated core lab toolchain is a comprehensive suite of connected software tools, workflows, and processes that manage the entire lifecycle of clinical trial imaging data - from acquisition and quality control to analysis and regulatory compliance. This unified approach eliminates silos between different stages of imaging management, reducing errors and accelerating trial timelines.
Key Components of Modern Core Lab Toolchains
The foundation of any effective clinical trial imaging operation relies on several interconnected components working in harmony. Understanding these elements is crucial for organizations looking to optimize their imaging workflows and ensure consistent, high-quality results across all trial sites.
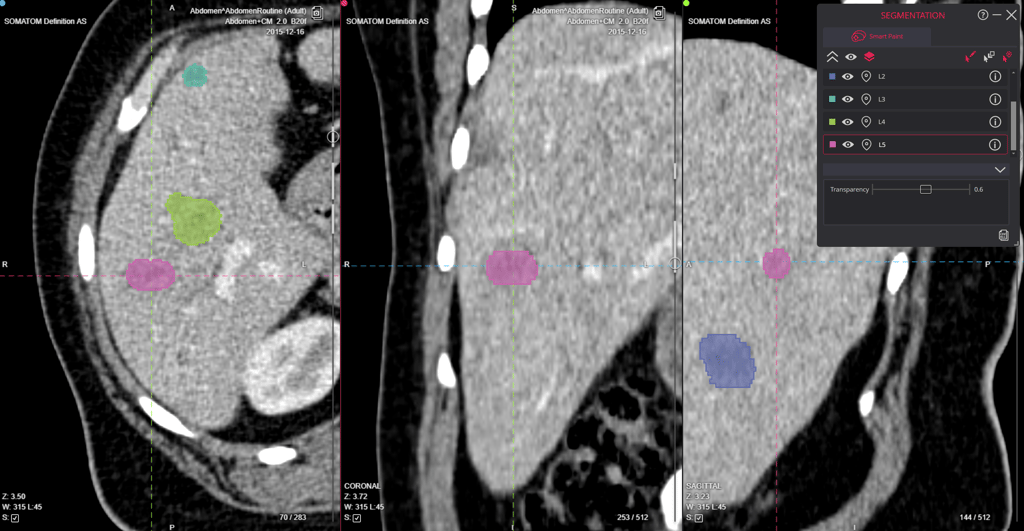
1. Image Acquisition and Standardization
Image acquisition forms the cornerstone of any clinical trial imaging process. The quality and consistency of initial data collection directly impact all subsequent analyses and results. Modern toolchains implement robust standardization protocols across all participating sites, ensuring uniform image quality and compatibility.
"Variations in imaging protocols, equipment, and techniques can introduce errors that undermine the integrity of study results. The core labs address these challenges by implementing standardized procedures for data acquisition, ensuring uniformity in image quality and interpretation."
confirms research published by Image Core Lab.
2. Automated Quality Control
Quality control in clinical trials requires meticulous attention to detail and consistent application of standards. Modern automated systems have transformed this traditionally manual process into a streamlined, reliable operation that catches issues early in the workflow and helps increasing efficiency and reducing trial costs and patient visits to the sites.
"A process that previously took days is reduced to minutes, and sites can receive feedback soon enough to take remedial action if needed, e.g. a patient re-scan. Additionally, the site itself can take on the QC burden using a supporting toolset."
states research published in PMC.
3. Data Management and Integration
The complexity of modern clinical trials demands sophisticated data management solutions. Today's integrated systems must handle massive volumes of imaging data while maintaining security, accessibility, integrity and compliance with various regulatory requirements.
Also Read: Imaging Data Management: Essential Strategies and Best Practices
Modern toolchains employ advanced system architectures and state-of-the-art technologies to create seamless data flows, enabling:
- Real-time data access and sharing
- Automated de-identification
- Standardized data formatting
- Secure cloud-based storage
4. Advanced Analytics Platform
The evolution of imaging analytics has revolutionized how we extract insights from clinical trial data. Modern platforms combine traditional statistical analysis with cutting-edge artificial intelligence to deliver deeper insights and faster results.
Also Read: Automated Clinical Trials Medical Imaging Platform
5. Regulatory Compliance Tools
In the highly regulated environment of clinical trials, compliance is not optional. Modern toolchains must incorporate robust compliance features that address multiple regulatory frameworks:
- 21 CFR Part 11 compliance
- GDPR data protection
- HIPAA privacy standards
- ICH GCP guidelines
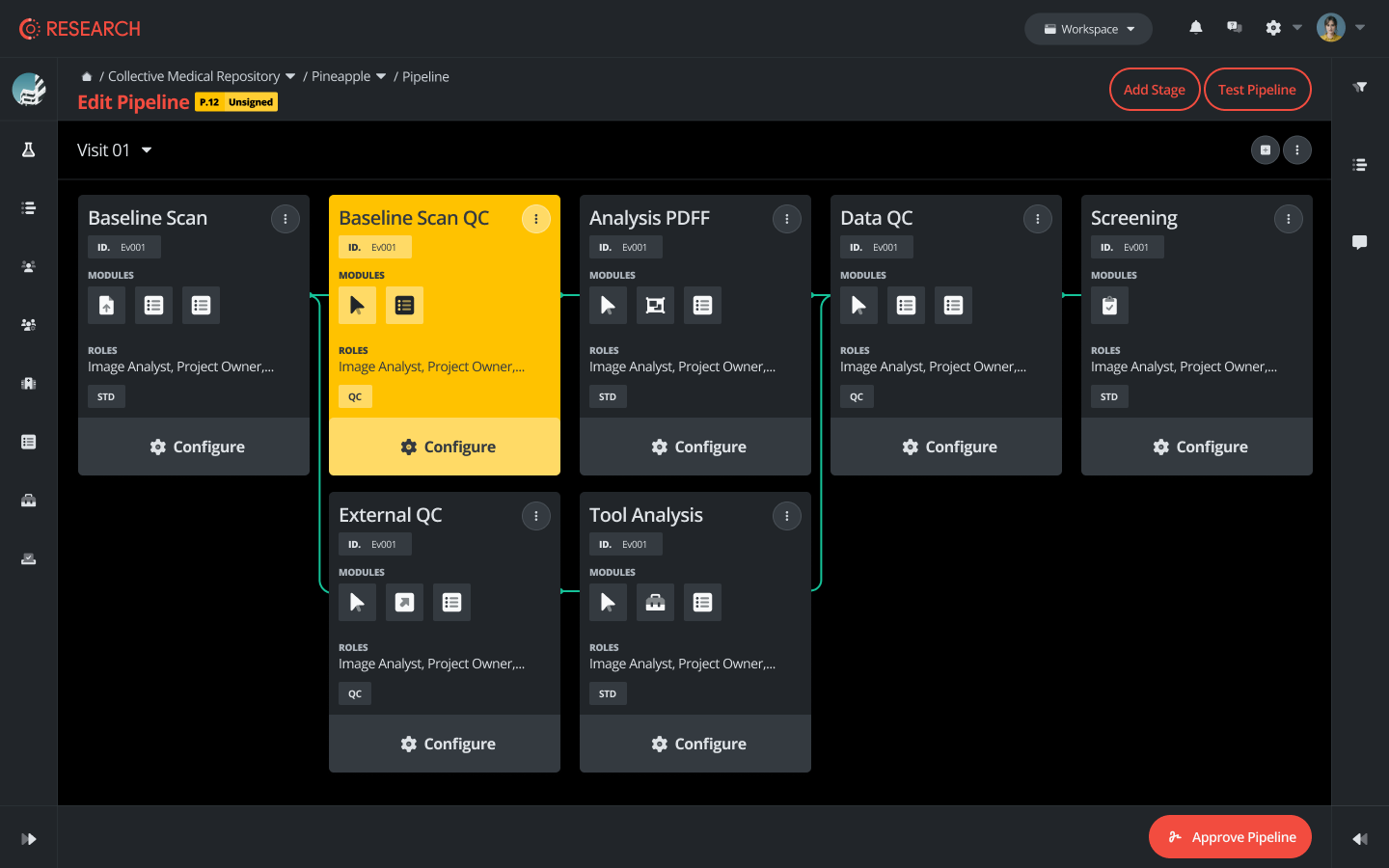
Advanced Workflow Automation
The evolution of clinical trial imaging has created a pressing need for more sophisticated workflow automation solutions. As trials become increasingly complex and data-intensive, manual processes can no longer keep pace with the demands of modern research. The integration of automated workflows represents a fundamental shift in how imaging core labs operate and manage their daily operations.
"The future of imaging clinical trials lies in intelligent automation that not only streamlines workflows but also enhances decision-making through advanced analytics. We're seeing a paradigm shift where automated systems can predict and prevent quality issues before they impact trial timelines,"
says Pär Kragsterman, CTO and Co-Founder of Collective Minds.
The latest generation of integrated toolchains, exemplified by platforms like Collective Minds Research, brings together multiple automation capabilities that transform traditional workflows. These systems provide comprehensive audit trails that document every interaction with the imaging data, ensuring complete transparency and traceability. Real-time quality monitoring systems automatically flag potential issues, allowing for immediate intervention and correction. This proactive approach significantly reduces the risk of downstream data quality issues that could impact trial outcomes.
Also Read: Medical Imaging Workflow: Optimize Clinical Trial Success
Best Practices for Implementation
The successful implementation of an integrated core lab toolchain requires careful planning and a systematic approach to change management. Organizations must first conduct a thorough assessment of their existing workflows, identifying bottlenecks and inefficiencies that could be addressed through automation. This initial evaluation should involve all stakeholders, from technicians and radiologists to IT staff and trial managers, ensuring that everyone's needs and concerns are considered in the implementation strategy.

A critical success factor is the development of a clear integration roadmap that outlines how the new toolchain will interface with existing systems and processes. This includes establishing data migration protocols, defining security requirements, and creating standard operating procedures that align with regulatory requirements. Organizations should also consider the scalability of their chosen solution, ensuring it can accommodate growing data volumes and increasing complexity of trial protocols.
Summary
An integrated core lab toolchain is essential for modern clinical trials, offering improved efficiency, data quality, and regulatory compliance. As the industry continues to evolve, solutions like Collective Minds Research are setting new standards for integrated imaging management, providing the tools and capabilities needed to accelerate clinical research while maintaining the highest quality standards.
FAQ
What makes a toolchain truly integrated?
A truly integrated toolchain provides seamless connectivity between all components, from image acquisition to analysis, with automated workflows and real-time data synchronization.
How does an integrated toolchain improve regulatory compliance?
Integrated toolchains include built-in compliance checks, audit trails, and validation processes that ensure adherence to regulatory requirements throughout the entire imaging workflow.
What role does AI play in modern core lab toolchains?
AI enhances toolchain capabilities through automated quality control, intelligent data analysis, and predictive insights, significantly reducing manual work and improving accuracy.
Reviewed by: Mathias Engström on March 20, 2025