Collective Minds Research

Welcome to your research workspace!
Collective Minds’ research workspace will help you improve the quality, speed, and delivery of your clinical research with imaging end-points. The research workspace helps you to organize your data, collaborators, analysis, and results. With research workspace and our legal framework, you can set up data sharing, including compliant data minimization, de-identification, and pseudonymization from a single institution or in a multi-site project.
Data sharing
Data sharing can be done retrospectively in bulk to our staging layer, using our proxy or for smaller studies using web upload. State-of-the-art repositories today are typically data/modality centric and handle a single data type very well. The Collective Minds research workspace implements a subject-centric perspective, where the data layers are wrapped around the subject, generating a solution where multi-modal data, structured by subject is instantly available to be found, analyzed, augmented, and used for next-generation data-driven medical imaging research.
Project design and pipeline
The project pipeline is the tool to design your project execution. Each pipeline is configurable with a set of events. Events are automatically executed or assigned to a project role, such as radiologist or scientist. Components to put in events are Data Upload, Data Browser, Quality Control, Input Form, Data Annotation and Containerized tool
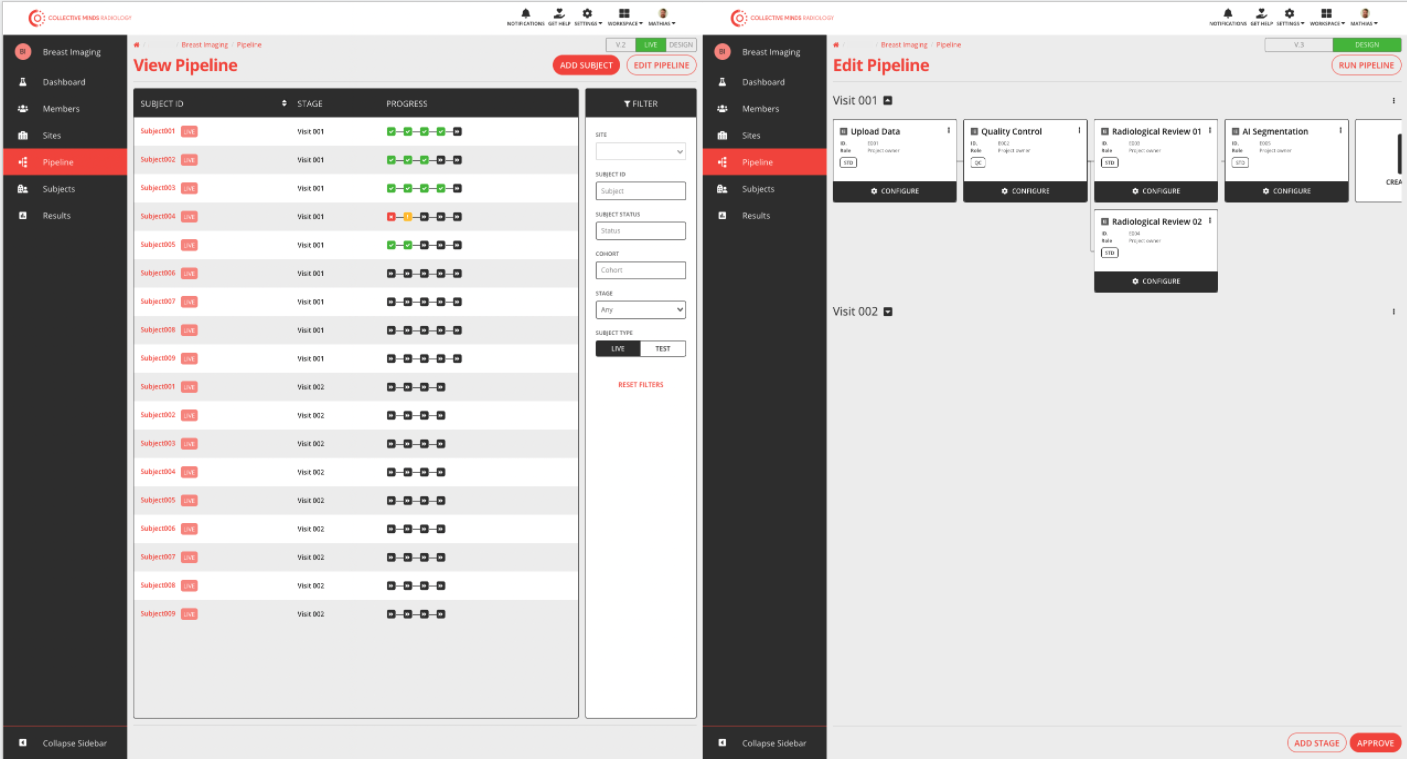
Project pipeline overview
Input Forms
Each repository has a library of input forms. An input can be designed to become a data transfer form, to be filled out during data transfer and check-in, or as a means of grading or classifying during data review.
Data classification and annotation
For reader, validation, and curation projects, having experts classify and annotate data is an important step. Using the tools in the research workspace, you can build specific events for this purpose.
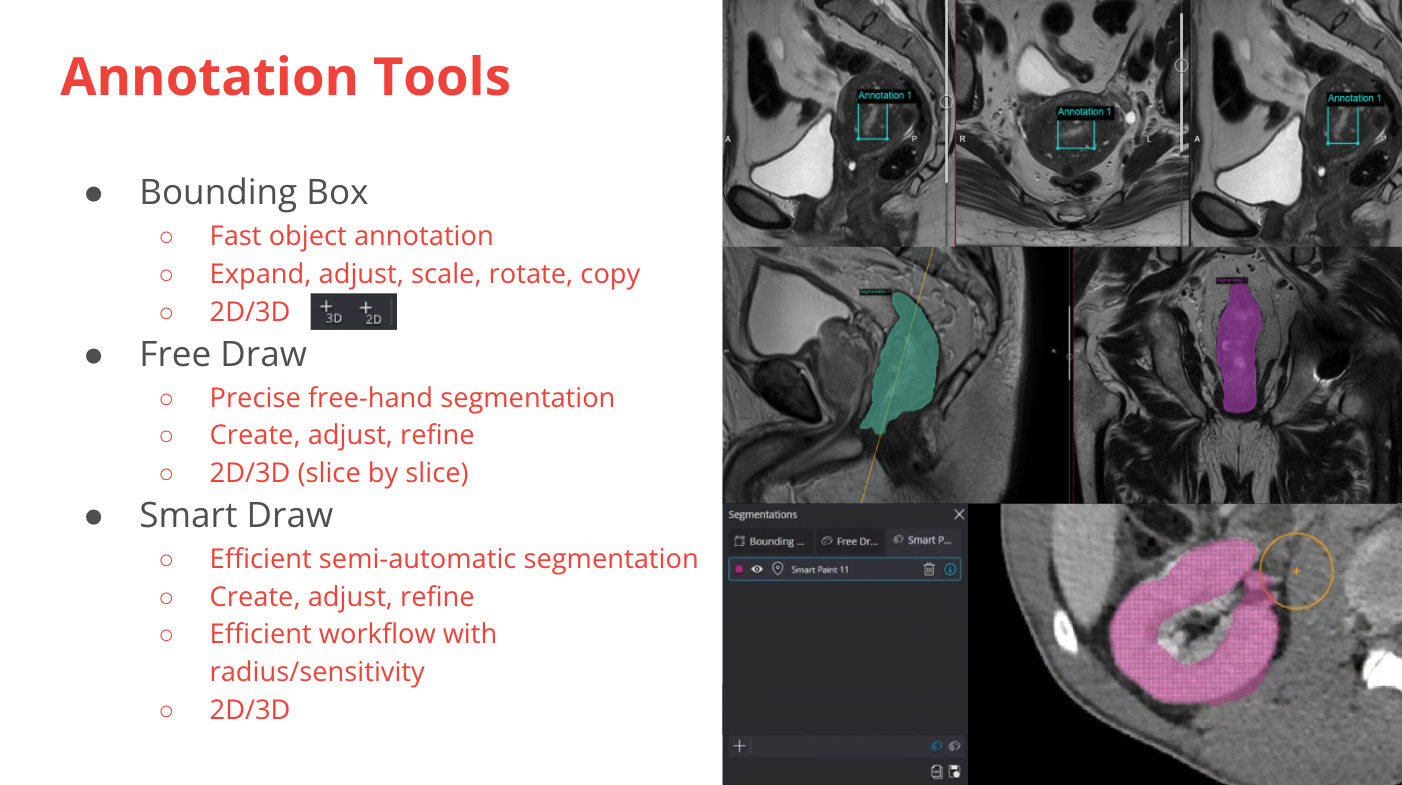
Annotation tools overview
Results
Any results generated by the pipeline can be assigned to be a project result. As the project progresses the results table is populated and can be delivered at any time of the project.
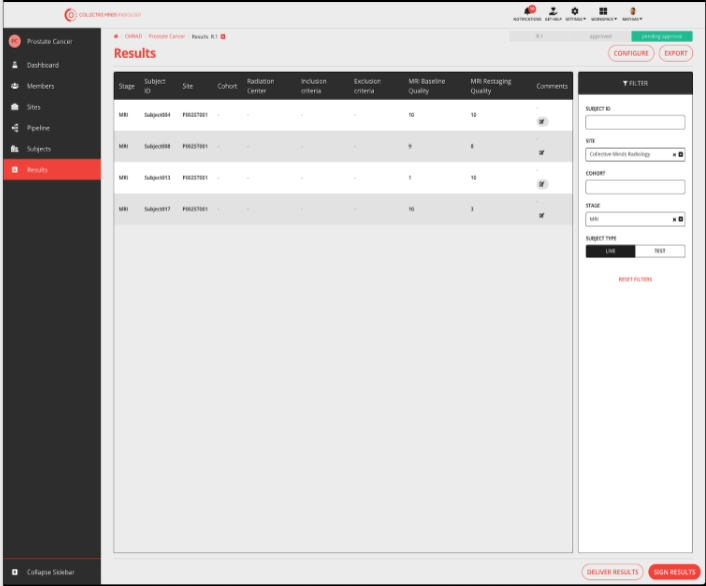
Results table
Audit trail
All interventions and changes to the project are registered in the audit trail. This gives powerful capabilities to audit projects, resolve issues, and give a full understanding of your project.
Research platform for academic research
Academic research of today is fast moving with rapid technology advancements and clinical experience. This entails the need for expert groups to collaborate, regardless of their location. As a result, we have created a platform where each participant can contribute with what they are best at and focus solely on that, from wherever they are. Regardless of whether data acquisition, curation, reading, processing, analysis, or project management is involved, the platform adapts to meet users’ needs.
We provide:
- simple and powerful tools for data upload, pseudonymization and ingestion where you are guided through the process securely
- review and reading tasks using a medical grade DICOM viewer and customizable case report forms
- annotation and segmentation tools directly in the viewer for data curation
- options for both local and on-platform processing
- version managed result tables
This occurs in a regulatory-compliant environment so that participant, subject and data integrity are protected.
Research platform for consortiums
Large research networks and consortiums provide the power and strength to tackle the most challenging questions in healthcare at scale. As diagnostic imaging is an integral part of modern medicine, the inclusion and processing of data from multiple modalities is becoming increasingly common. From Collective Minds we acknowledge that cross-institutional international collaboration is challenging and involves adaptation and flexibility to match each participant’s needs and requirements. This both from a practical and regulatory sense. Data needs to be efficiently ingested, harmonized, curated, processed, and controlled to be as valuable as possible. With this in mind, Collective Minds have created an adaptive and scalable solution for networks and consortiums.
We provide:
- all the functionality that is available for the academic researcher
- retrospective and prospective multimodal radiological, pathology, genomics and clinical data ingestion paths
- large scale data storage, processing and interfacing
- granular access control for participants and collaborators
- a neutral environment for data processing without binary sharing requirements
- consultancy in regulatory questions
We are happy and willing to participate in both the partnership and supplier roles as needed for the project.
Research platform for product validation
The Collective Minds research platform is an excellent environment for emerging products to be validated. Comparative studies can be conducted efficiently with both internal and external contributors using a cost-effective process. To minimize undesired overhead work, external consultants are given easy access and direct tasks to minimize ambiguity. Throughout the entire life of the product validation, traceability and reproducibility are ensured without compromising the integrity of the study. We offer the possibility to integrate and connect functionality to support all stages of product development.
We provide:
- All the functionality that is available for the academic researcher
- Integration support for external products and processing
- Training and support for internal and external users
- A multiphase and pilot project approach to study management
- Easy methods for importing and exporting data
You can rely on us to assist, accelerate, and help you move your next product toward market.
Research platform for sponsored research
Based on GAMP5 compliant development practices and QMS according to ISO 13485, the Collective Minds platform is designed from the ground up to support good clinical research practice. For contract research organizations, we provide a complete and cost effective solution for conducting clinical studies and providing support for clinical trials on any scale. With compliance and privacy by design, risks can be mitigated and actions are traceable by a comprehensive audit trail. As a standard feature, the platform offers electronic case report forms and digital signatures as well as a medical-grade DICOM viewer that supports annotation and segmentation tools. For processing and quality assurance in-house developed tools and functions can be embedded using versioning and local validation.
Want to hear more? Book a demo with us using the form below. We look forward to talking to you.