.png?width=1915&height=1436&name=Rectangle%20(2).png)
Scientists in medical imaging often encounter barriers in data sharing, and access during collaborative research.
Managing project workflow and controlling access for project members across institutions is challenging and time consuming. Traditional research methods within the confines of individual institutions can hinder progress and limit the scope of studies.
Collective Minds Research for Academia is designed to enhance medical imaging research by providing a global platform for seamless collaboration across institutions.
Clinical Research Data Management Software
- Intuitive Interface.
- Seamless Integration.
- Efficient Data sharing.
- Scalability and flexibility.
- Data Security and Confidentiality.
- eCRFs
.png?width=1500&height=1125&name=Edit%20Pipeline-Galaxy%20Tab%20S8%20Ultra%20(2).png)

The system was very easy to use. Everything went really smoothly.

Prof. Jussi Hirvonen
Tampere University
Intuitive Interface
Research for Academia's platform is designed with user-friendliness at its core, ensuring an accessible and streamlined experience for all users, regardless of technical expertise. By simplifying data entry and management, the interface minimizes the learning curve and enhances user efficiency. Key features, including customizable templates, drag-and-drop functionality, and real-time data validation, are integrated to significantly boost productivity and optimize the overall user experience.

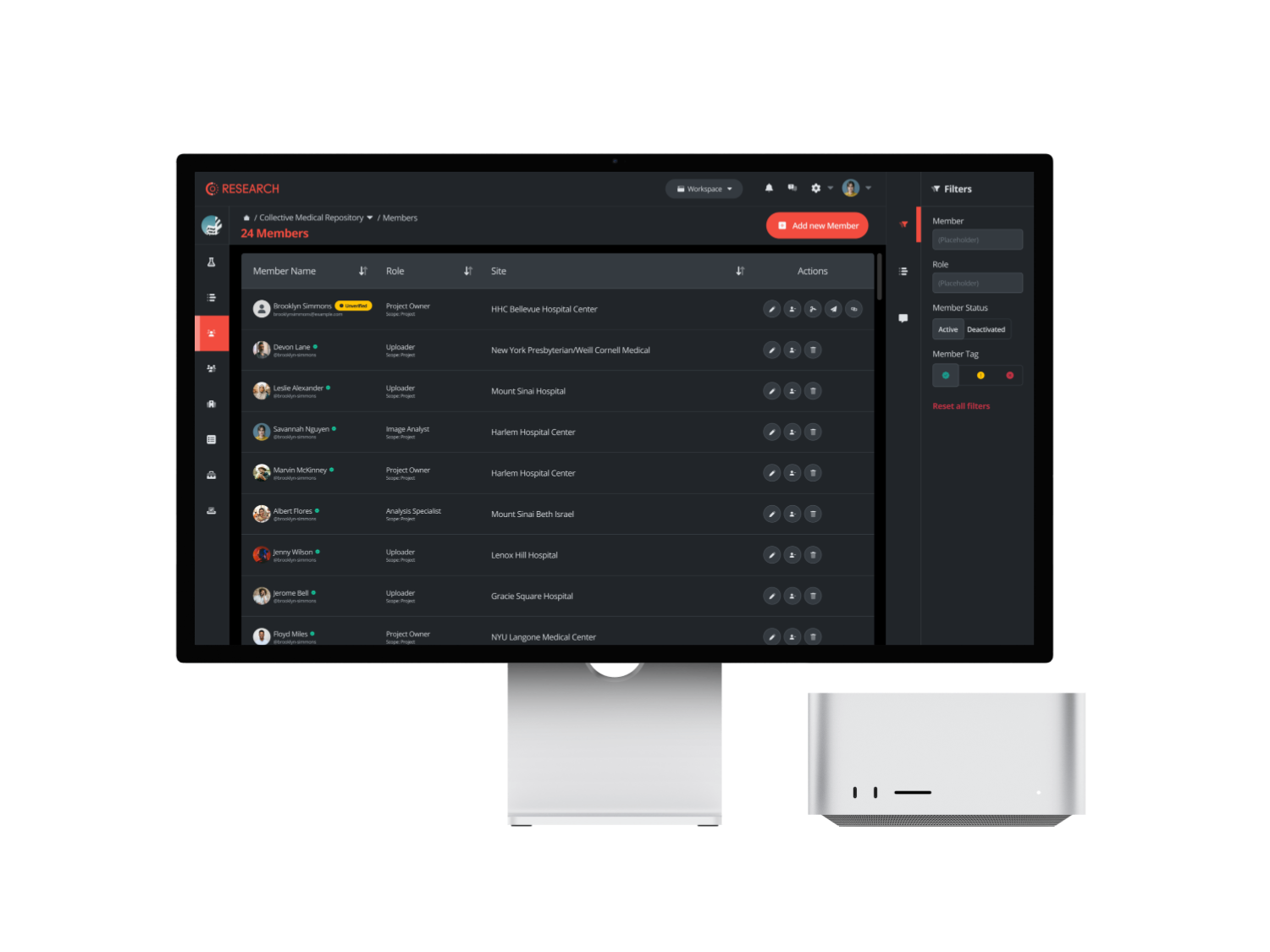
Seamless Integration to your PACS
With CM Connect, seamlessly integrate your hospital or local systems to enable automatic sharing of DICOM studies. The platform ensures that all data is securely de-identified and pseudonymized before it leaves your local environment, facilitating quicker and easier data sharing while maintaining strict confidentiality standards.
Scalability and flexibility
Collective Minds Research for Academia is designed to scale effortlessly, accommodating growth and adapting to evolving needs. It offers flexible, customizable workflows that ensure the system remains responsive and adaptable to changing demands.
.png?width=1500&height=1125&name=Research-Results-Galaxy%20Tab%20S8%20Ultra%20(3).png)

Easy, intuitive, and after just some minutes of training, the team was ready to go." The clear design of the solution had also a very motivating and encouraging effect on the reader."

Dr. Anselm Schulz
Oslo University Hospital
.png?width=300&height=300&name=shield-security%20(2).png)
Data Security and Confidentiality
Collective Minds provides a secure processing environment. The platform has been designed to comply with the strongest worldwide regulations and data protection laws, such as GDPR, and with the most stringent information security standards like ISO 27001.
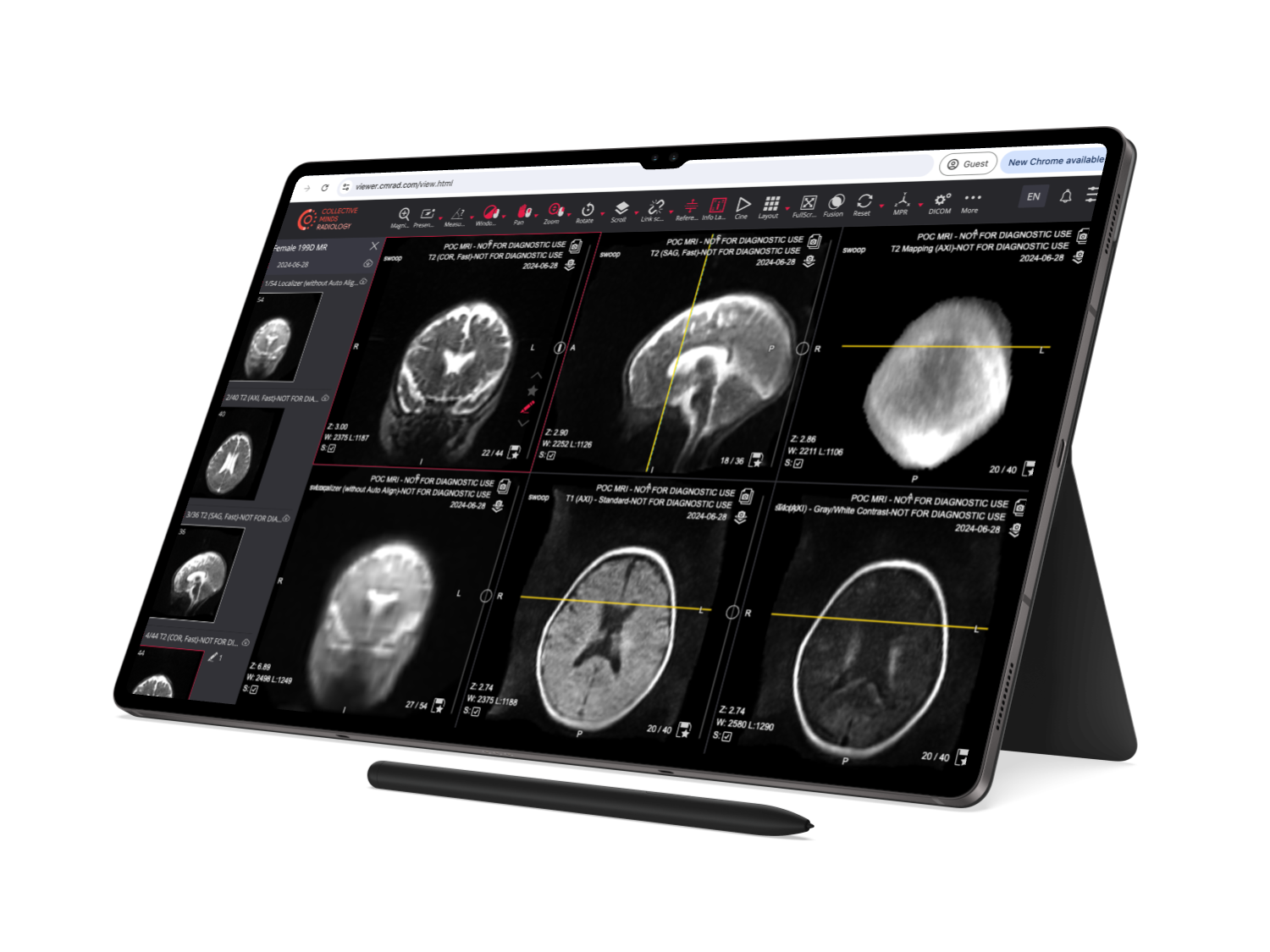
Clinical-grade DICOM viewer
The visualization tool includes, among others:
- Segmentation and Annotation
- Presenter tools with fading arrows and draws
- Advance measurement tools
- PET-CT, Mr and NM Fusion
- Histogram
- Calibration line
- Ruler y Text Annotations
- MPR: Orthogonal, Axial, Coronal, Sagittal, MISTs
- CINE
- Window Level
- Crosshair
- MPR Oblique MIST feature with MPR/MIP/3D rendering.
- Reference lines
- Align and Lock
- Sync Windowing.

eCRFs (Electronic Case Report Forms)
+2000
Researchers
+400
Research projects
+200
Institutions connected directly to Collective Minds
Academic studies for clinical researchers
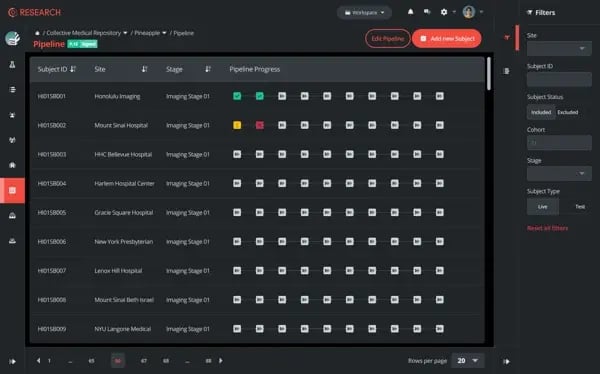
- Seamlessly gather data from collaborating institutions, share it globally, and receive input from readers worldwide.
- Design projects using the pipeline tool, assigning events to dedicated roles.
- Customize events with tools like data upload, clinical-grade DICOM image review, and eCRF assessments.
- All project members have personalized task lists aligned with the project pipeline.
- Maintain control over access permissions, ensuring oversight of project progress for all subjects, anytime.
.png?width=1500&height=1125&name=Research-Results-Galaxy%20Tab%20S8%20Ultra%20(3).png)
Clinical audit and validation projects for hospitals
Run your internal or external radiology clinical audit by allowing internal or external expert to review your reports and cases according to specific quality criteria.
Did the radiology report correctly mention the relevant findings?
Was the clinical significance of those findings clearly communicated?
We provide a comprehensive service for clinical audits including access to sub-speciality radiology experts.
More than 200 Institutions trust Collective Minds









Frequently Asked Questions
What can I use Collective Minds Research for Academia for?
- Medical imaging research
- Reader studies
- Clinical trials
- Research consortia
- Clinical audits
- AI-validation studies
Why should I use Collective Minds Research for Academia?
Collective Minds is a clinically robust platform, supporting secure data collection and collaboration across multiple sites. Whether you’re organizing a multi-institution research consortium or need a flexible tool for managing imaging data, Collective Minds provides the features and compliance framework needed for academic research.
See also: What are the benefits of using Collective Minds Research for Academia?
What specialties can it be used for?
Collective Minds is suitable for any medical specialty or modality to facilitate your research within medical imaging. The clinical grade dicom viewer supports all clinical imaging modalities, providing all the commonly used tools normally available in a hospital PACS
What are the benefits of using Collective Minds Research for Academia?
Whenever you want to collect data from multiple institutions or make a data set available to project members around the world, Collective Minds is the perfect platform to facilitate your project.
The platform also facilitates project steering through roles, tasks, and permissions, allowing you to manage the activities and members of your project effectively.
The versioning and logging of all activities ensures compliance with regulatory requirements and saves you a lot of documentation work!
How fast can I start to use Collective Minds Research for Academia?
As soon as you are a customer of Collective Minds Research you will have your own repository and can start setting up your project(s) right away.
If you are not a customer yet, but curious to see for your self how it works, send an email to support@cmrad.com, and we will provide you with access to our Customer Playground, to get the "look-and-feel" for yourself
Are there customizable privacy settings to control access to sensitive information?
Access to the data in your project/repository is controlled through roles and permissions, ensuring full control over what the different members can access. In addition all activities are logged and versioned, ensuring full transparency. Personal sensitive information is not stored on Collective Minds.
What are the financial options available for purchasing and maintaining your platform?
The most common financial option is a subscription-based model, i.e., a monthly cost for the platform access. Payments can be made on a yearly, quarterly, or monthly basis.
In some cases, a project cost is more suitable than a monthly fee.
Contact sales@cmrad.com for more information.
Do you offer flexible pricing plans based on our usage needs and budget constraints?
Yes, the pricing is based on the customer's needs. We offer various tiers of pricing based on the scope. Reach out to sales@cmrad.com for more details regarding pricing options.
Are there additional costs for scaling our usage or adding more users?
How does your platform handle collaboration among users and teams?
Are there tools for real-time collaboration, such as video conferencing or shared document editing?
We don’t offer a built-in video conferencing tool, but you can utilize any third-party service of your choice. Real-time collaborative editing for documents is not available; however, you can store and share documents within the platform, controlling access in the same way you would for imaging data.
Can users from different institutions collaborate seamlessly on projects or research?
Absolutely. You can assign roles and site affiliations for collaborators from any institution, making multi-institutional research straightforward.
What integration options do you offer with other educational or research tools?
Is your platform compatible with our existing IT infrastructure and software?
CMRAD platform is cloud-based and accessible as long as you are connected to the internet."
How easy is it to migrate our current data and workflows onto your platform?
1. Connect: This option allows you to push cases directly from your PACS system.
2. Web Upload: Upload cases individually and directly into the pipeline when stored locally on your computer.
3. Bulk Transfer via sFTP: As a premium service, we can handle ingesting your data into the platform through bulk transfer.
Existing workflows will need to build the pipeline directly within the system."
What level of technical support do you provide, and what are the response times?
The deployment engineers will assist you with the installation of CMRAD Connect. The Customer Success team will support you with project configurations and platform features, tailored to the specifics of your agreement. Our office hours are Monday through Friday, from 9 AM to 5 PM. Collective Minds Service Level Agreement.
Are there training resources or support materials available to help onboard our team?
help.cmrad.com
How frequently do you update your platform, and how do you handle feature requests?
Our platform undergoes regular updates, including major feature releases several times a year and ongoing critical patches and security enhancements.
We prioritize feature requests based on a structured process considering client feedback, market trends, and technical feasibility. Feature requests are evaluated based on their potential impact and alignment with our roadmap. Our release announcements and newsletters keep clients informed about major updates and new features.
Can we customize the platform to meet specific institutional or departmental needs?
What analytics and reporting capabilities does your platform offer?
How do you ensure the long-term reliability and sustainability of your platform?
We prioritize platform reliability and sustainability through a robust, multi-layered approach.
Our scalable cloud infrastructure enables dynamic resource allocation. Automated monitoring and alerting systems proactively identify and mitigate potential issues.
Regular code and infrastructure reviews, adherence to industry best practices, and rigorous testing and internal controls ensure platform stability. Our comprehensive business continuity and disaster recovery plan safeguards data integrity and platform resilience.
How does your platform comply with the GDPR or other laws and regulations regarding data protection and privacy?
Collective Minds is designed to empower healthcare and research institutions by providing a secure and compliant platform for data sharing. We address the critical challenges of safeguarding patient privacy and ensuring data protection while enabling collaboration. Rooted in privacy by design, our platform offers a secure processing environment that adheres to the highest privacy standards, including the General Data Protection Regulation (GDPR).
As a dedicated Data Processor, we manage data strictly on behalf of healthcare and research institutions, ensuring it is used only for authorized research purposes and under their explicit instructions. Our platform incorporates a comprehensive framework of legal commitments, technical controls, and operational safeguards to maintain a trusted and compliant environment for data processing.
Our innovative solution, CM Connect (link), ensures that data is encrypted and pseudonymized within the institution or hospital before it is shared with our cloud. This guarantees that personal data remains protected during transfer and processing while aligning with local and EU-level privacy requirements. Once the data enters our secure cloud environment, it benefits from uniform, robust security measures that facilitate further processing in a legally compliant and ethically sound manner.
With Collective Minds, you can confidently collaborate and share data for your research projects while safeguarding patient privacy and personal data protection at every step.
What measures do you have in place to protect our data and intellectual property?
Can you provide details on where our data will be stored and how it will be secured?
AWS was chosen for its unmatched security, scalability, and global data availability, essential for a multinational clinical data sharing platform. It holds globally recognized certifications, including ISO 27001, ISO 27017, ISO 27018, SOC 1/2/3, NIS2, GDPR, and HIPAA, ensuring robust security and data protection practices.
In Frankfurt, AWS employs server replicability across multiple Availability Zones, ensuring data remains secure and accessible even in the event of server failure. The platform is also designed to meet local legal requirements worldwide (e.g., Australia, Canada, India, the US, and Europe) while maintaining consistent, stringent compliance standards across the global platform.
Our infrastructure, which includes encryption, firewalls, and continuous monitoring, ensures high availability, low latency, and fault tolerance, making it ideal for secure healthcare collaboration.
Data compliance
.png?width=300&height=300&name=shield-security%20(2).png)



